
ISO 15223-1
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements

ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements

ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements
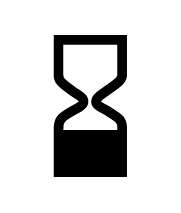
ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements

ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements

ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements
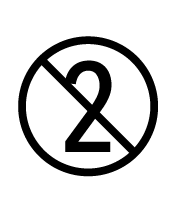
ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements

ISO 15223-1:2012 (Annex B, 15223-1:2012)
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements

ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements

ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements



ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements
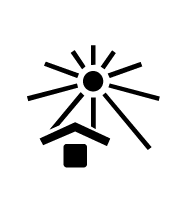
ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements
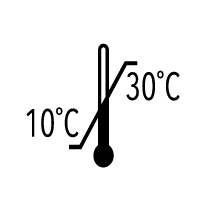
ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements
ASTM F739 & ASTM D6978
Symbol used for SW chemotherapy tested gloves that have been tested either by ASTM F739 or by ASTM D6978.
ASTM D6978-05 (2019)
Symbol used for SW fentanyl & simulated gastric acid tested gloves that have been tested by ASTM D6978-05 (2019).

SHA Approved
The Skin Health Alliance award professional accreditation and endorsement to companies, services and brands seeking specialist independent dermatological recognition for their product research.

BSIF Registered Safety Supplier
The United Kingdom's leading trade body within the safety industry.

REACH Compliant
REACH is a regulation of the European Union, adopted to improve the protection of human health and the environment from the risks that can be posed by chemicals, while enhancing the competitiveness of the EU chemicals industry.

TR CU 019/2011
Safety Personal Protection Equipment

UL NFPA 1999
Standard on Protective Clothing and Ensembles for Emergency Medical Operations

ISO 15223-1:2012
Medical devices -- Symbols to be used with medical device labels, labelling and information to be supplied -- Part 1: General requirements
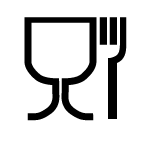
EU 1935/2004
Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food

American National Standard For Hand Protection Classification
ANSI/ISEA 105-2016 addresses the classification and testing of hand protection for specific performance properties related to chemical and industrial applications.

American National Standard For Hand Protection Classification
ANSI/ISEA 105-2016 addresses the classification and testing of hand protection for specific performance properties related to chemical and industrial applications.

American National Standard For Hand Protection Classification
ANSI/ISEA 105-2016 addresses the classification and testing of hand protection for specific performance properties related to chemical and industrial applications.

ANSI/ISEA 138-2019: American National Standard For Performance And Classification For Impact-Resistant Gloves

Type A
Protective gloves against dangerous chemicals and micro-organisms
The chemicals break through the glove material at a molecular level. The breakthrough time is here evaluated and the glove must withstand a breakthrough time of at least: Type A – 30 minutes (level 2) against minimum 6 test chemicals

Type B
Protective gloves against dangerous chemicals and micro-organisms
The chemicals break through the glove material at a molecular level. The breakthrough time is here evaluated and the glove must withstand a breakthrough time of at least: Type B – 30 minutes (level 2) against minimum 3 test chemicals

Type C
Protective gloves against dangerous chemicals and micro-organisms
The chemicals break through the glove material at a molecular level. The breakthrough time is here evaluated and the glove must withstand a breakthrough time of at least: Type C – 10 minutes (level 1) against minimum 1 test chemical

Protective gloves against dangerous chemicals and micro-organisms — Part 5: Terminology and performance requirements for micro-organisms risks
EN 374-5:2016
All gloves must be tested against micro-organisms. The gloves are tested to protect against bacteria and fungi, but also viruses if requested, according to EN 374-5:2016.

Protective gloves against dangerous chemicals and micro-organisms — Part 5: Terminology and performance requirements for micro-organisms risks
EN 374-5:2016
All gloves must be tested against micro-organisms. The gloves are tested to protect against bacteria and fungi, but also viruses if requested, according to EN 374-5:2016.

EN 388:2016+A1:2018
Protective gloves. General requirements and test methods

EN 16350:2016
Prescribes test conditions and minimum requirements for the electrostatic properties of protective gloves

EN511:2016
Measure how well the glove can withstand both convective cold and contact cold

(made in compliance with Regulation (EU) 2016/425, Category III MODULE C2)
Regulation (EU) 2016/425
Personal Protective Equipment Directive. Notified Body ID is 0493.

Regulation (EU) 2016/425
Personal Protective Equipment Directive. Notified Body ID is 0598.

(made in compliance with 93/42/EEC)
Medical Device Regulation (MDR) (2017/745)
Medical Device Regulation (MDR) (2017/745). Medical Device Directive.


The UKCA (UK Conformity Assessed) marking is a new UK product marking that is used for goods being placed on the market in Great Britain (England, Wales and Scotland).